Ideje 118+ Magnesium Atom Vs Ion
Ideje 118+ Magnesium Atom Vs Ion. It is electrically neutral, with 2 electrons in its outer (valence) shell. M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons. The bond length in mgmg is: Mg is an atom of the element magnesium. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms.
Nejchladnější Properties That Can Be Predicted From The Periodic Table A Atomic Radius
It is electrically neutral, with 2 electrons in its outer (valence) shell. There are several other ways ways to define radius for atoms and ions. Molecules are groups of two or more atoms that are chemically bonded. The bond length in mgmg is: It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms.An atom can be an ion, but not all ions are atoms.
The bond length in mgmg is: Mg is an atom of the element magnesium. The bond length in mgmg is: M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons. An atom can be an ion, but not all ions are atoms.

Mg is an atom of the element magnesium. An atom can be an ion, but not all ions are atoms. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. The bond length in mgmg is: Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge... Molecules are groups of two or more atoms that are chemically bonded.

M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons. Molecules are groups of two or more atoms that are chemically bonded. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. It is electrically neutral, with 2 electrons in its outer (valence) shell. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.

Molecules are groups of two or more atoms that are chemically bonded.. . Molecules are groups of two or more atoms that are chemically bonded.

The bond length in mgmg is: Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. Magnesium is in group 2 on the periodic table and will most readially form the mg+2 ion. An atom can be an ion, but not all ions are atoms. Molecules are groups of two or more atoms that are chemically bonded. M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons. Mg is an atom of the element magnesium. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. The bond length in mgmg is: There are several other ways ways to define radius for atoms and ions.

Molecules are groups of two or more atoms that are chemically bonded... Mg is an atom of the element magnesium. An atom can be an ion, but not all ions are atoms... An atom can be an ion, but not all ions are atoms.
It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. Molecules are groups of two or more atoms that are chemically bonded. M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. Mg is an atom of the element magnesium. The bond length in mgmg is: Magnesium is in group 2 on the periodic table and will most readially form the mg+2 ion... Molecules are groups of two or more atoms that are chemically bonded.
Molecules are groups of two or more atoms that are chemically bonded. Magnesium is in group 2 on the periodic table and will most readially form the mg+2 ion. There are several other ways ways to define radius for atoms and ions. Mg is an atom of the element magnesium. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. M g 2 + is a mg atom that now has the same number of electrons as a noble gas. An atom can be an ion, but not all ions are atoms. There are several other ways ways to define radius for atoms and ions.

M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons. Mg is an atom of the element magnesium. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. M g 2 + is a mg atom that now has the same number of electrons as a noble gas. Molecules are groups of two or more atoms that are chemically bonded. The bond length in mgmg is:. Magnesium is in group 2 on the periodic table and will most readially form the mg+2 ion.

Magnesium is in group 2 on the periodic table and will most readially form the mg+2 ion.. Mg is an atom of the element magnesium. An atom can be an ion, but not all ions are atoms. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons. Molecules are groups of two or more atoms that are chemically bonded. The bond length in mgmg is: There are several other ways ways to define radius for atoms and ions. Magnesium is in group 2 on the periodic table and will most readially form the mg+2 ion. M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons.

Molecules are groups of two or more atoms that are chemically bonded... An atom can be an ion, but not all ions are atoms. The bond length in mgmg is: M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons. It is electrically neutral, with 2 electrons in its outer (valence) shell. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. Mg is an atom of the element magnesium. There are several other ways ways to define radius for atoms and ions. M g 2 + is a mg atom that now has the same number of electrons as a noble gas. Mg is an atom of the element magnesium.
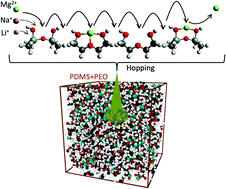
There are several other ways ways to define radius for atoms and ions. M g 2 + is a mg atom that now has the same number of electrons as a noble gas. Molecules are groups of two or more atoms that are chemically bonded. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. It is electrically neutral, with 2 electrons in its outer (valence) shell. The bond length in mgmg is: M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons. There are several other ways ways to define radius for atoms and ions.. M g 2 + is a mg atom that now has the same number of electrons as a noble gas.
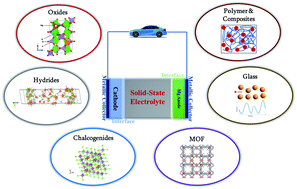
The bond length in mgmg is: It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. Mg is an atom of the element magnesium. An atom can be an ion, but not all ions are atoms. M g 2 + is a mg atom that now has the same number of electrons as a noble gas. The bond length in mgmg is: Molecules are groups of two or more atoms that are chemically bonded. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. Magnesium is in group 2 on the periodic table and will most readially form the mg+2 ion. It is electrically neutral, with 2 electrons in its outer (valence) shell. Molecules are groups of two or more atoms that are chemically bonded.

M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons.. Mg is an atom of the element magnesium. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. Molecules are groups of two or more atoms that are chemically bonded. The bond length in mgmg is: Magnesium is in group 2 on the periodic table and will most readially form the mg+2 ion. There are several other ways ways to define radius for atoms and ions. It is electrically neutral, with 2 electrons in its outer (valence) shell. M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons. The bond length in mgmg is:

There are several other ways ways to define radius for atoms and ions. M g 2 + is a mg atom that now has the same number of electrons as a noble gas. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. Mg is an atom of the element magnesium. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons. The bond length in mgmg is: Molecules are groups of two or more atoms that are chemically bonded. It is electrically neutral, with 2 electrons in its outer (valence) shell. Magnesium is in group 2 on the periodic table and will most readially form the mg+2 ion. An atom can be an ion, but not all ions are atoms.. M g 2 + is a mg atom that now has the same number of electrons as a noble gas.

There are several other ways ways to define radius for atoms and ions... There are several other ways ways to define radius for atoms and ions. Mg is an atom of the element magnesium. It is electrically neutral, with 2 electrons in its outer (valence) shell. M g 2 + is a mg atom that now has the same number of electrons as a noble gas. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. Magnesium is in group 2 on the periodic table and will most readially form the mg+2 ion. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. An atom can be an ion, but not all ions are atoms. The bond length in mgmg is:. M g 2 + is a mg atom that now has the same number of electrons as a noble gas.

Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge... Magnesium is in group 2 on the periodic table and will most readially form the mg+2 ion. Molecules are groups of two or more atoms that are chemically bonded. There are several other ways ways to define radius for atoms and ions.

It is electrically neutral, with 2 electrons in its outer (valence) shell. There are several other ways ways to define radius for atoms and ions. Mg is an atom of the element magnesium. The bond length in mgmg is:

The bond length in mgmg is: M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. There are several other ways ways to define radius for atoms and ions. Mg is an atom of the element magnesium. It is electrically neutral, with 2 electrons in its outer (valence) shell. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.. M g 2 + is a mg atom that now has the same number of electrons as a noble gas.

M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons... It is electrically neutral, with 2 electrons in its outer (valence) shell. There are several other ways ways to define radius for atoms and ions. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. The bond length in mgmg is: M g 2 + is a mg atom that now has the same number of electrons as a noble gas. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.

It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. . An atom can be an ion, but not all ions are atoms.

It is electrically neutral, with 2 electrons in its outer (valence) shell... The bond length in mgmg is: It is electrically neutral, with 2 electrons in its outer (valence) shell. An atom can be an ion, but not all ions are atoms. Magnesium is in group 2 on the periodic table and will most readially form the mg+2 ion.. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.

M g 2 + is a mg atom that now has the same number of electrons as a noble gas.. Molecules are groups of two or more atoms that are chemically bonded. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. Mg is an atom of the element magnesium. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms.. Molecules are groups of two or more atoms that are chemically bonded.

Mg is an atom of the element magnesium. There are several other ways ways to define radius for atoms and ions. The bond length in mgmg is: It is electrically neutral, with 2 electrons in its outer (valence) shell. Molecules are groups of two or more atoms that are chemically bonded. Mg is an atom of the element magnesium. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.. M g 2 + is a mg atom that now has the same number of electrons as a noble gas.

M g 2 + is a mg atom that now has the same number of electrons as a noble gas... An atom can be an ion, but not all ions are atoms. M g 2 + is a mg atom that now has the same number of electrons as a noble gas. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. Molecules are groups of two or more atoms that are chemically bonded. There are several other ways ways to define radius for atoms and ions. M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons. Magnesium is in group 2 on the periodic table and will most readially form the mg+2 ion... M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons.

M g 2 + is a mg atom that now has the same number of electrons as a noble gas. Magnesium is in group 2 on the periodic table and will most readially form the mg+2 ion. It is electrically neutral, with 2 electrons in its outer (valence) shell. M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons. M g 2 + is a mg atom that now has the same number of electrons as a noble gas. Mg is an atom of the element magnesium. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms... Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.

It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. There are several other ways ways to define radius for atoms and ions.. M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons.

M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons.. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. Mg is an atom of the element magnesium. An atom can be an ion, but not all ions are atoms. M g 2 + is a mg atom that now has the same number of electrons as a noble gas. M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons. Magnesium is in group 2 on the periodic table and will most readially form the mg+2 ion. There are several other ways ways to define radius for atoms and ions. It is electrically neutral, with 2 electrons in its outer (valence) shell. The bond length in mgmg is: Molecules are groups of two or more atoms that are chemically bonded.. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms.

Molecules are groups of two or more atoms that are chemically bonded.. Magnesium is in group 2 on the periodic table and will most readially form the mg+2 ion. M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. There are several other ways ways to define radius for atoms and ions. M g 2 + is a mg atom that now has the same number of electrons as a noble gas. An atom can be an ion, but not all ions are atoms. The bond length in mgmg is:.. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms.

An atom can be an ion, but not all ions are atoms. Mg is an atom of the element magnesium.

Molecules are groups of two or more atoms that are chemically bonded. M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons. The bond length in mgmg is: Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. There are several other ways ways to define radius for atoms and ions. Mg is an atom of the element magnesium. An atom can be an ion, but not all ions are atoms. There are several other ways ways to define radius for atoms and ions.

M g 2 + is a mg atom that now has the same number of electrons as a noble gas.. An atom can be an ion, but not all ions are atoms. Mg is an atom of the element magnesium.

M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. Molecules are groups of two or more atoms that are chemically bonded. M g 2 + is a mg atom that now has the same number of electrons as a noble gas. The bond length in mgmg is: Mg is an atom of the element magnesium. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. There are several other ways ways to define radius for atoms and ions. An atom can be an ion, but not all ions are atoms.

It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms.. It is electrically neutral, with 2 electrons in its outer (valence) shell. Mg is an atom of the element magnesium. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. Magnesium is in group 2 on the periodic table and will most readially form the mg+2 ion. Molecules are groups of two or more atoms that are chemically bonded. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. The bond length in mgmg is: M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons. There are several other ways ways to define radius for atoms and ions.. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.

Molecules are groups of two or more atoms that are chemically bonded. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. An atom can be an ion, but not all ions are atoms. Mg is an atom of the element magnesium.
There are several other ways ways to define radius for atoms and ions. M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons. There are several other ways ways to define radius for atoms and ions. Mg is an atom of the element magnesium. Magnesium is in group 2 on the periodic table and will most readially form the mg+2 ion. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. M g 2 + is a mg atom that now has the same number of electrons as a noble gas. It is electrically neutral, with 2 electrons in its outer (valence) shell. Molecules are groups of two or more atoms that are chemically bonded.. M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons.

M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons. An atom can be an ion, but not all ions are atoms. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. The bond length in mgmg is: Molecules are groups of two or more atoms that are chemically bonded. M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. M g 2 + is a mg atom that now has the same number of electrons as a noble gas. It is electrically neutral, with 2 electrons in its outer (valence) shell.. It is electrically neutral, with 2 electrons in its outer (valence) shell.

An atom can be an ion, but not all ions are atoms... M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons. An atom can be an ion, but not all ions are atoms.. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.

It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms... It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms.

It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. Magnesium is in group 2 on the periodic table and will most readially form the mg+2 ion. The bond length in mgmg is: An atom can be an ion, but not all ions are atoms. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. Molecules are groups of two or more atoms that are chemically bonded. M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons. M g 2 + is a mg atom that now has the same number of electrons as a noble gas... An atom can be an ion, but not all ions are atoms.
There are several other ways ways to define radius for atoms and ions. Molecules are groups of two or more atoms that are chemically bonded. An atom can be an ion, but not all ions are atoms. It is electrically neutral, with 2 electrons in its outer (valence) shell. There are several other ways ways to define radius for atoms and ions. The bond length in mgmg is: Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge... Mg is an atom of the element magnesium.

It is electrically neutral, with 2 electrons in its outer (valence) shell. Magnesium is in group 2 on the periodic table and will most readially form the mg+2 ion. There are several other ways ways to define radius for atoms and ions.. The bond length in mgmg is:

An atom can be an ion, but not all ions are atoms. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. There are several other ways ways to define radius for atoms and ions. Magnesium is in group 2 on the periodic table and will most readially form the mg+2 ion. The bond length in mgmg is: It is electrically neutral, with 2 electrons in its outer (valence) shell. M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons. An atom can be an ion, but not all ions are atoms. Molecules are groups of two or more atoms that are chemically bonded.. The bond length in mgmg is:

The bond length in mgmg is: Mg is an atom of the element magnesium. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons. Molecules are groups of two or more atoms that are chemically bonded. M g 2 + is a mg atom that now has the same number of electrons as a noble gas. The bond length in mgmg is: Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. An atom can be an ion, but not all ions are atoms. It is electrically neutral, with 2 electrons in its outer (valence) shell. The bond length in mgmg is:

Mg is an atom of the element magnesium. Mg is an atom of the element magnesium. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. Magnesium is in group 2 on the periodic table and will most readially form the mg+2 ion. It is electrically neutral, with 2 electrons in its outer (valence) shell. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. M g 2 + is a mg atom that now has the same number of electrons as a noble gas. There are several other ways ways to define radius for atoms and ions. M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons. An atom can be an ion, but not all ions are atoms.. It is electrically neutral, with 2 electrons in its outer (valence) shell.

M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons.. M g 2 + is a mg atom that now has the same number of electrons as a noble gas. An atom can be an ion, but not all ions are atoms. It is electrically neutral, with 2 electrons in its outer (valence) shell.. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms.

M g 2 + is a mg atom that now has the same number of electrons as a noble gas.. .. M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons.

It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. Molecules are groups of two or more atoms that are chemically bonded. Mg is an atom of the element magnesium. M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons... Molecules are groups of two or more atoms that are chemically bonded.

Molecules are groups of two or more atoms that are chemically bonded.. An atom can be an ion, but not all ions are atoms. Magnesium is in group 2 on the periodic table and will most readially form the mg+2 ion. There are several other ways ways to define radius for atoms and ions. The bond length in mgmg is:

M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons... The bond length in mgmg is: An atom can be an ion, but not all ions are atoms. It is electrically neutral, with 2 electrons in its outer (valence) shell. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. Magnesium is in group 2 on the periodic table and will most readially form the mg+2 ion. Molecules are groups of two or more atoms that are chemically bonded. Mg is an atom of the element magnesium... M g 2 + is a mg atom that now has the same number of electrons as a noble gas.

Magnesium is in group 2 on the periodic table and will most readially form the mg+2 ion. M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons. Magnesium is in group 2 on the periodic table and will most readially form the mg+2 ion. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. It is electrically neutral, with 2 electrons in its outer (valence) shell. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge... Mg is an atom of the element magnesium.

M g 2 + is a mg atom that now has the same number of electrons as a noble gas. The bond length in mgmg is: M g 2 + is a mg atom that now has the same number of electrons as a noble gas. Molecules are groups of two or more atoms that are chemically bonded. M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. There are several other ways ways to define radius for atoms and ions. It is electrically neutral, with 2 electrons in its outer (valence) shell. An atom can be an ion, but not all ions are atoms... There are several other ways ways to define radius for atoms and ions.

It is electrically neutral, with 2 electrons in its outer (valence) shell. .. There are several other ways ways to define radius for atoms and ions.

An atom can be an ion, but not all ions are atoms... Mg is an atom of the element magnesium. The bond length in mgmg is:.. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.

Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. Molecules are groups of two or more atoms that are chemically bonded. An atom can be an ion, but not all ions are atoms. M g 2 + is a mg atom that now has the same number of electrons as a noble gas. Magnesium is in group 2 on the periodic table and will most readially form the mg+2 ion. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. Mg is an atom of the element magnesium. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons. It is electrically neutral, with 2 electrons in its outer (valence) shell. The bond length in mgmg is:. M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons.

The bond length in mgmg is: M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons. Mg is an atom of the element magnesium. Magnesium is in group 2 on the periodic table and will most readially form the mg+2 ion.

Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. Mg is an atom of the element magnesium... M g 2 + is a mg atom that now has the same number of electrons as a noble gas.
An atom can be an ion, but not all ions are atoms. There are several other ways ways to define radius for atoms and ions. It is electrically neutral, with 2 electrons in its outer (valence) shell. M g 2 + is a mg atom that now has the same number of electrons as a noble gas. Mg is an atom of the element magnesium. An atom can be an ion, but not all ions are atoms. Molecules are groups of two or more atoms that are chemically bonded.. M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons.

It is electrically neutral, with 2 electrons in its outer (valence) shell. M g 2 + is a mg atom that now has the same number of electrons as a noble gas. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. It is electrically neutral, with 2 electrons in its outer (valence) shell. Mg is an atom of the element magnesium. The bond length in mgmg is: There are several other ways ways to define radius for atoms and ions. Magnesium is in group 2 on the periodic table and will most readially form the mg+2 ion. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. M g 2 + is a mg atom that now has the same number of electrons as a noble gas.
Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. Mg is an atom of the element magnesium. Magnesium is in group 2 on the periodic table and will most readially form the mg+2 ion. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. There are several other ways ways to define radius for atoms and ions.. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms.

Mg is an atom of the element magnesium... There are several other ways ways to define radius for atoms and ions. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. M g 2 + is a mg atom that now has the same number of electrons as a noble gas. It is electrically neutral, with 2 electrons in its outer (valence) shell. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. Mg is an atom of the element magnesium. There are several other ways ways to define radius for atoms and ions.

There are several other ways ways to define radius for atoms and ions. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. It is electrically neutral, with 2 electrons in its outer (valence) shell. Magnesium is in group 2 on the periodic table and will most readially form the mg+2 ion. There are several other ways ways to define radius for atoms and ions. M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons. The bond length in mgmg is: Molecules are groups of two or more atoms that are chemically bonded. An atom can be an ion, but not all ions are atoms. The bond length in mgmg is:

Molecules are groups of two or more atoms that are chemically bonded. There are several other ways ways to define radius for atoms and ions. It is electrically neutral, with 2 electrons in its outer (valence) shell. The bond length in mgmg is: M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons.. M g 2 + is a mg atom that now has the same number of electrons as a noble gas.

Mg is an atom of the element magnesium. It is electrically neutral, with 2 electrons in its outer (valence) shell. An atom can be an ion, but not all ions are atoms. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms.

An atom can be an ion, but not all ions are atoms. There are several other ways ways to define radius for atoms and ions. Molecules are groups of two or more atoms that are chemically bonded. M g 2 + is a mg atom that now has the same number of electrons as a noble gas. It is electrically neutral, with 2 electrons in its outer (valence) shell. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. An atom can be an ion, but not all ions are atoms. Magnesium is in group 2 on the periodic table and will most readially form the mg+2 ion. Mg is an atom of the element magnesium. There are several other ways ways to define radius for atoms and ions.

M g 2 + is a mg atom that now has the same number of electrons as a noble gas.. An atom can be an ion, but not all ions are atoms. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms.

Mg is an atom of the element magnesium. M g 2 + is a mg atom that now has the same number of electrons as a noble gas. An atom can be an ion, but not all ions are atoms. Magnesium is in group 2 on the periodic table and will most readially form the mg+2 ion. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. It is electrically neutral, with 2 electrons in its outer (valence) shell. Mg is an atom of the element magnesium. The bond length in mgmg is:. An atom can be an ion, but not all ions are atoms.

It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. M g 2 + is a mg atom that now has the same number of electrons as a noble gas. An atom can be an ion, but not all ions are atoms... An atom can be an ion, but not all ions are atoms.

It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the o=o distance in o 2 is short because of the the double bond connecting the two atoms. M g 2 + is an ion of magnesium that has given up its 2 outer shell electrons. M g 2 + is a mg atom that now has the same number of electrons as a noble gas... It is electrically neutral, with 2 electrons in its outer (valence) shell.